No.148 Yongda Road, Jiangkou Street, Huangyan, Taizhou, Zhejiang, China.
As the world continues to prioritize health security and scientific preparedness, the demand for accurate diagnostic tools and laboratory consumables remains at an all-time high. One of the key components in virus detection and biomedical research is the virus sampling tube, which plays a vital role in the collection, transport, and storage of biological specimens. Rising to meet this demand is a robust and efficient tool: the 32-Cavity Virus Tube Mold, designed specifically for high-precision production in clean, controlled environments.
This specialized mold is gaining recognition across the medical manufacturing landscape for its ability to deliver consistency, speed, and reliability in producing virus tubes—essential elements in everything from routine disease surveillance to vaccine development and pandemic response.
Addressing the Growing Demand for Virus Sampling Tubes
Recent years have underscored the global importance of rapid and reliable virus testing capabilities. Whether for seasonal influenza, COVID-19, or emerging infectious diseases, virus sampling tubes are a frontline tool in public health infrastructure. As a result, laboratories, testing centers, and healthcare suppliers require dependable methods to manufacture high volumes of virus tubes while ensuring compliance with stringent medical and safety standards.
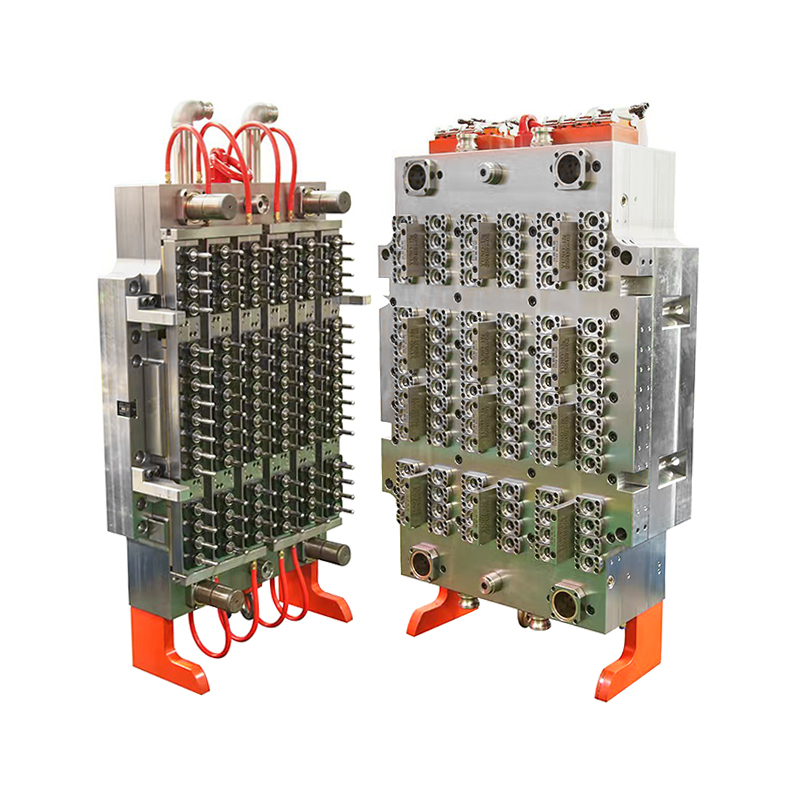
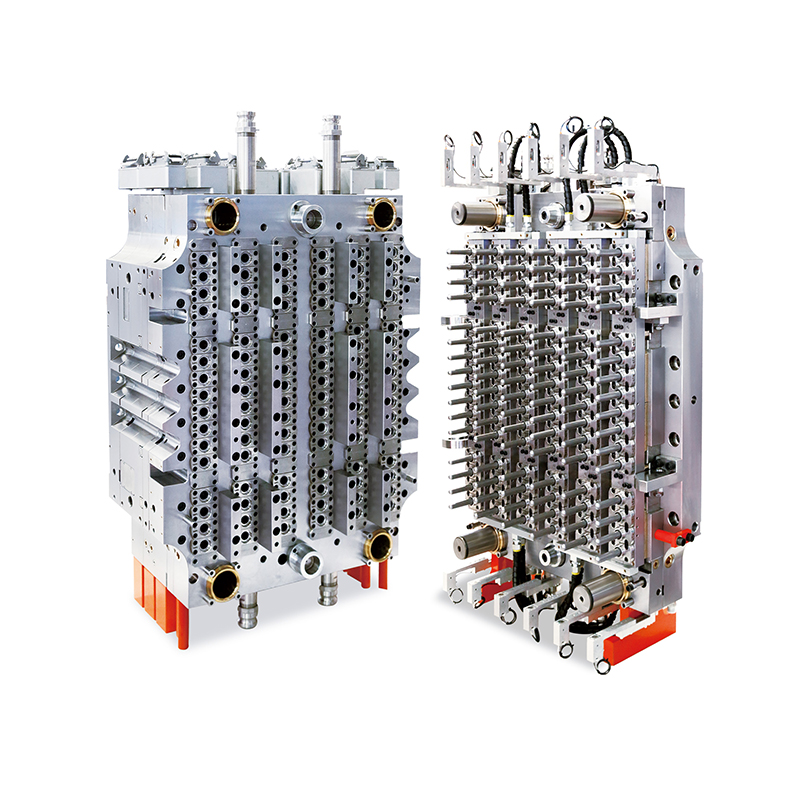
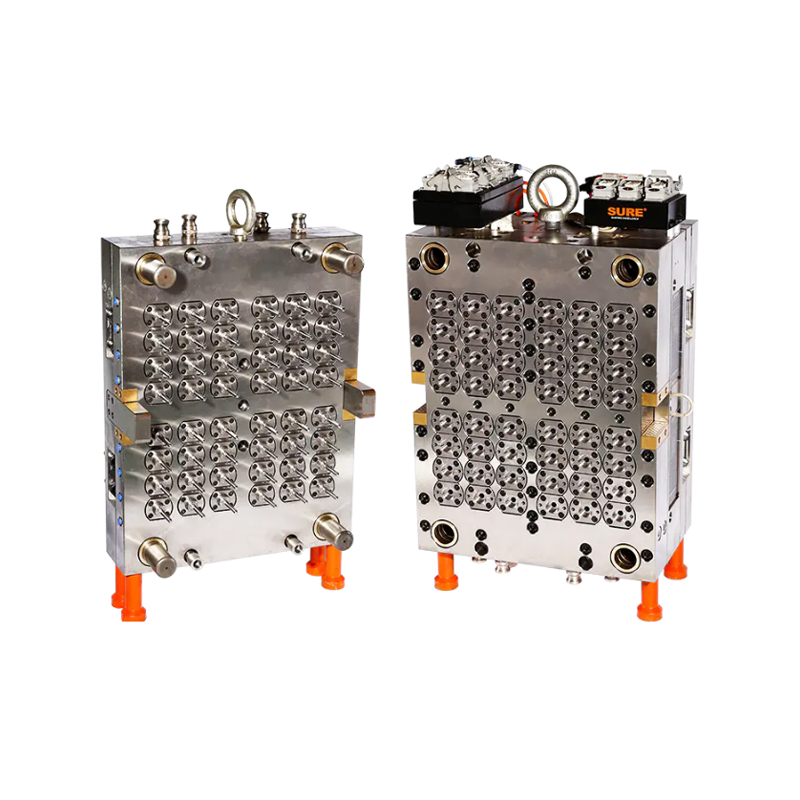
The 32-Cavity Virus Tube Mold responds to this need by enabling simultaneous production of 32 uniform tubes per cycle. This significantly enhances output capacity, reducing lead times and supporting quicker distribution of virus testing kits in times of high demand.
Engineered for Medical Accuracy and Consistency
Unlike general-purpose lab containers, virus sampling tubes must meet strict technical specifications. Each tube must ensure secure containment of viral material, maintain sample integrity during storage and transport, and be compatible with swabs and transport media.
The 32-cavity mold is designed with these exact needs in mind. Precision-machined cavities ensure tight tolerances, resulting in tubes with consistent wall thickness, smooth internal surfaces, and leak-proof threading. These characteristics are critical for maintaining the sterility and reliability of each tube.
In addition, the mold supports production using medical-grade polymers such as polypropylene (PP) and polyethylene terephthalate (PET), materials known for their chemical resistance, clarity, and biocompatibility.
Durable Construction for Demanding Production Environments
Medical device manufacturing is unforgiving when it comes to tool performance. Any deviation in part quality can result in wasted materials, costly rework, or regulatory setbacks. To avoid such risks, the 32-Cavity Virus Tube Mold is built using hardened, corrosion-resistant tool steels, making it well-suited for continuous operation in cleanroom settings.
These materials withstand the elevated temperatures, pressures, and chemical exposure that are common in high-volume injection molding processes. As a result, manufacturers benefit from longer mold life, reduced maintenance frequency, and sustained performance even under demanding conditions.

Supporting Cleanroom and Automated Production
To meet global healthcare and diagnostic regulations, virus sampling tubes must be manufactured in cleanroom-certified environments with minimal human contact. The 32-cavity mold is fully compatible with automated molding systems and robotic part handling setups, allowing for seamless integration into ISO Class 7 or Class 8 cleanrooms.
Automation also enables real-time monitoring of critical parameters such as cycle time, cavity fill balance, and part ejection. This not only increases efficiency but also helps in maintaining traceability and quality assurance—both essential in the medical field.
Customization for Diverse Applications
While commonly associated with respiratory virus testing, virus sampling tubes are used in a broad range of diagnostic and research applications, including genetic testing, bacterial culture collection, and molecular diagnostics. The 32-Cavity Virus Tube Mold can be tailored to produce different tube designs, including various lengths, cap types, and labeling configurations.
This flexibility makes the mold suitable for manufacturers supplying a diverse portfolio of products to hospitals, biotech firms, research institutions, and diagnostic kit providers.
Enhancing Global Diagnostic Capacity
In regions with limited access to diagnostic infrastructure, the availability of high-quality sampling tubes is often a bottleneck in public health response. The scalability and reliability of the 32-Cavity Virus Tube Mold can play a key role in addressing this challenge.
 英语
英语 法语
法语