No.148 Yongda Road, Jiangkou Street, Huangyan, Taizhou, Zhejiang, China.
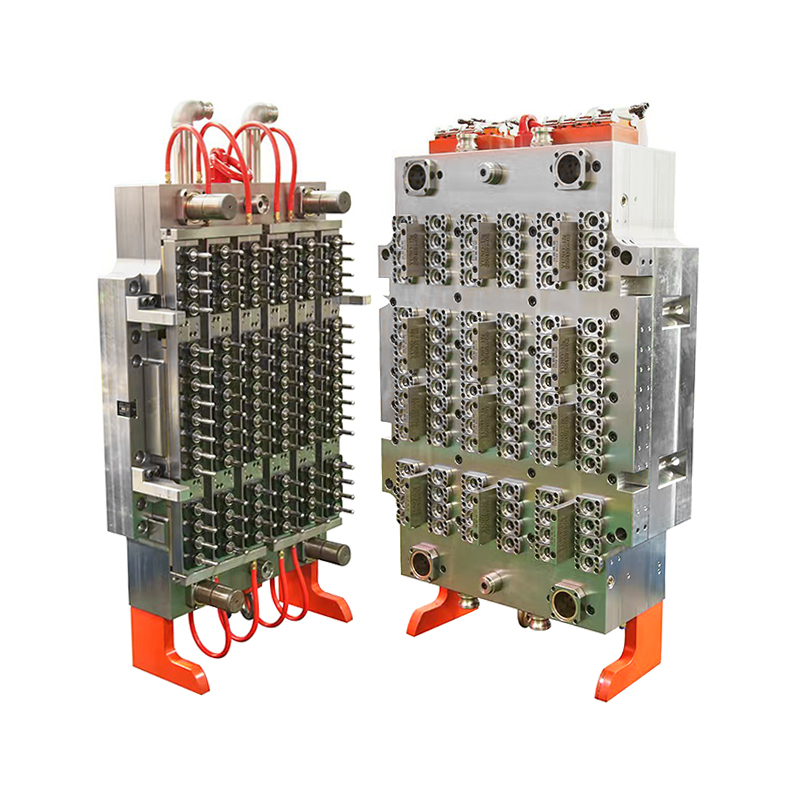
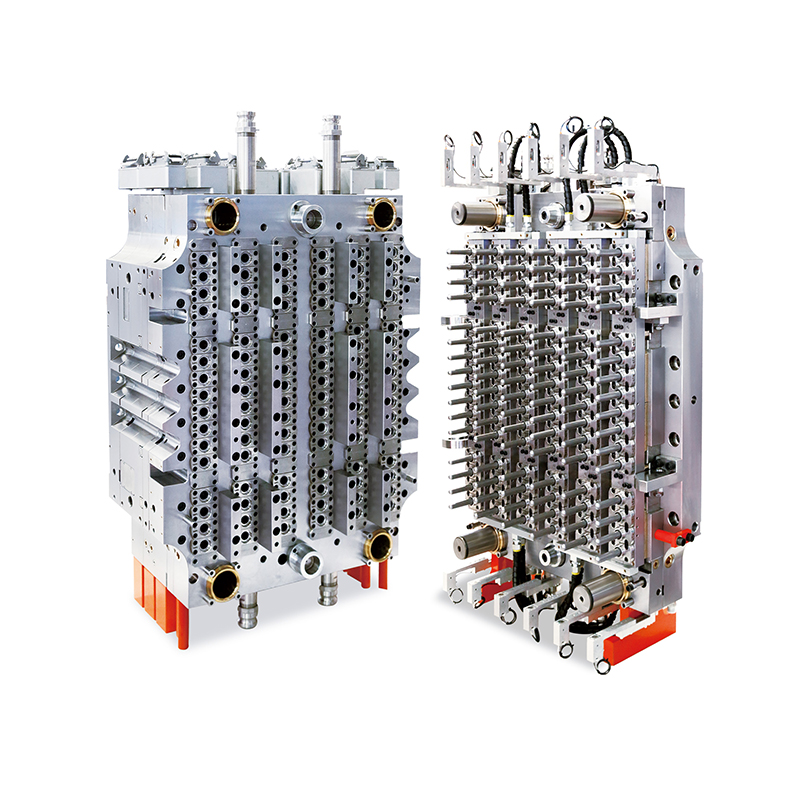
In the world of medical device production, precision, sterility, and material compatibility are non-negotiable requirements. As healthcare demand increases globally, so too does the need for reliable, high-volume manufacturing tools that can support the production of critical consumables. Among the essential of these are blood collection tubes, which are used daily in laboratories, hospitals, and diagnostic centers worldwide. Now, a new generation of tooling is setting the standard: the 48-Cavity Blood Collection Tube Mold.
This specialized mold design is gaining significant traction in the medical manufacturing sector due to its ability to produce blood collection tubes at scale with high accuracy and repeatability, while ensuring material integrity and compliance with strict industry standards.
Meeting the Growing Demand for Medical Consumables
The global diagnostics and laboratory testing market is experiencing steady growth, driven by increased healthcare access, aging populations, and ongoing public health monitoring. As a result, the demand for blood collection tubes—both vacuum and non-vacuum types—has risen substantially. Manufacturers are under pressure to boost production rates without sacrificing quality, safety, or regulatory compliance.
The 48-cavity mold provides a strong solution. By enabling the simultaneous formation of 48 identical blood collection tubes in each molding cycle, the tool dramatically improves throughput. This level of productivity is essential for companies supplying millions of units per month to healthcare systems around the world.
Precision Engineering for Critical Tolerances
Unlike typical consumer packaging, blood collection tubes require micron-level precision to ensure safe use with needles, vacuum seals, and automated lab equipment. The dimensional accuracy of these tubes is critical for maintaining internal pressure, preventing contamination, and ensuring compatibility with lab instruments and centrifuges.
The 48-Cavity Blood Collection Tube Mold is engineered to meet these exacting standards. It incorporates high-precision machining techniques, uniform cavity design, and advanced temperature regulation to ensure that every tube produced conforms to strict specifications. This includes consistent inner diameters, wall thicknesses, and smooth finishes that allow seamless integration with stoppers, caps, and labeling processes.
Material Compatibility and Chemical Resistance
Blood collection tubes are often made from medical-grade PET, polypropylene, or glass-like plastics, all of which must withstand exposure to various reagents and anticoagulants during use. The mold must be equally resistant to these substances during production.
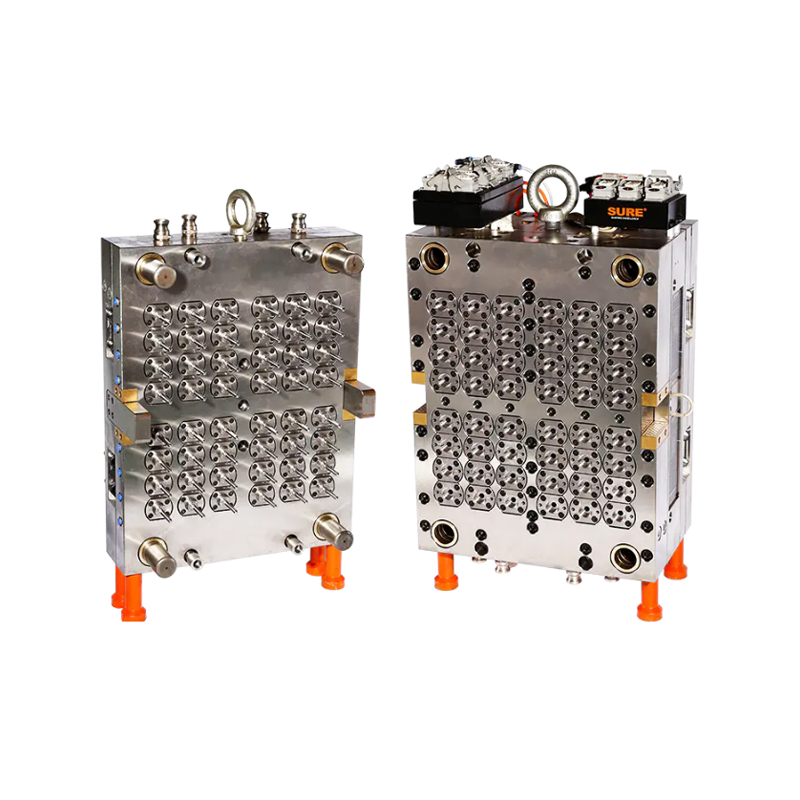
To meet this need, the 48-cavity mold is constructed using chemically resistant, high-performance alloys and surface treatments. These materials ensure that the tool can operate reliably even in environments where chemical exposure is frequent. This not only protects the mold from corrosion and wear but also prevents any contamination of the medical-grade materials used in tube manufacturing.
Long-Term Durability for Continuous Operation
Production of blood collection tubes often takes place in cleanroom environments operating 24/7 to meet global demand. Under such conditions, durability and operational stability are essential.
The 48-cavity mold is built with reinforced tool steel and modular components that extend its service life while allowing for efficient maintenance and part replacement. Precision alignment systems ensure smooth cavity performance throughout long production runs, reducing the risk of downtime due to misalignment or mechanical failure.
This translates into lower maintenance costs and reduced interruptions, which are critical for medical manufacturers that rely on continuous production schedules.
Integration with Cleanroom and Automated Systems
In medical device manufacturing, maintaining sterility and minimizing human intervention are top priorities. The 48-Cavity Blood Collection Tube Mold is designed for full compatibility with automated injection molding machines and robotic handling systems, including automated tube ejection, cooling, and packaging.
Additionally, the mold supports ISO Class 7 and Class 8 cleanroom environments, ensuring that tubes can be produced in sterile or near-sterile conditions. This is particularly important for manufacturers serving hospitals and labs with high biosafety requirements.
 英语
英语 法语
法语