No.148 Yongda Road, Jiangkou Street, Huangyan, Taizhou, Zhejiang, China.
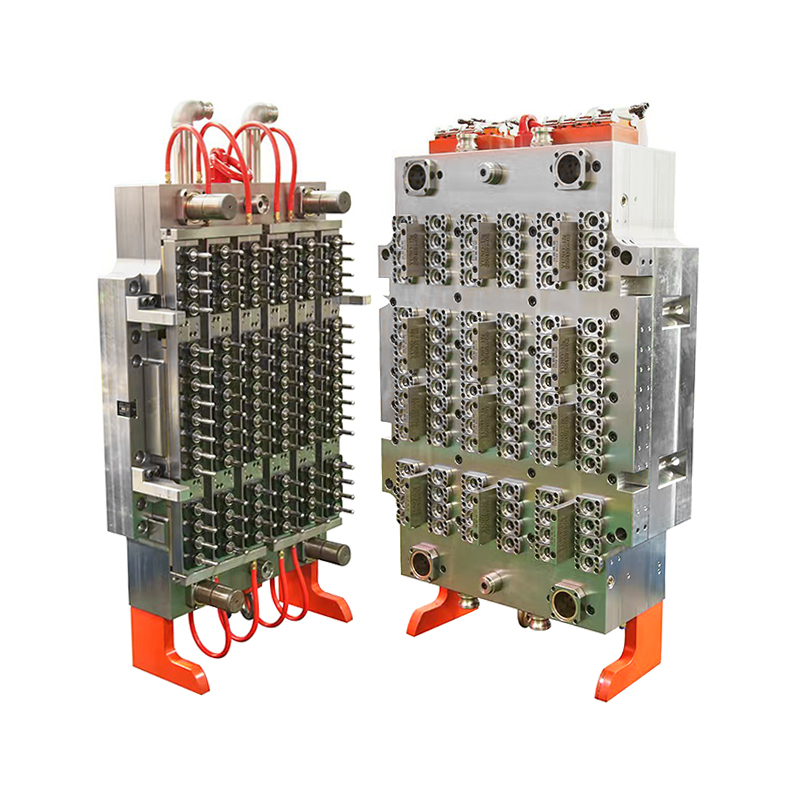
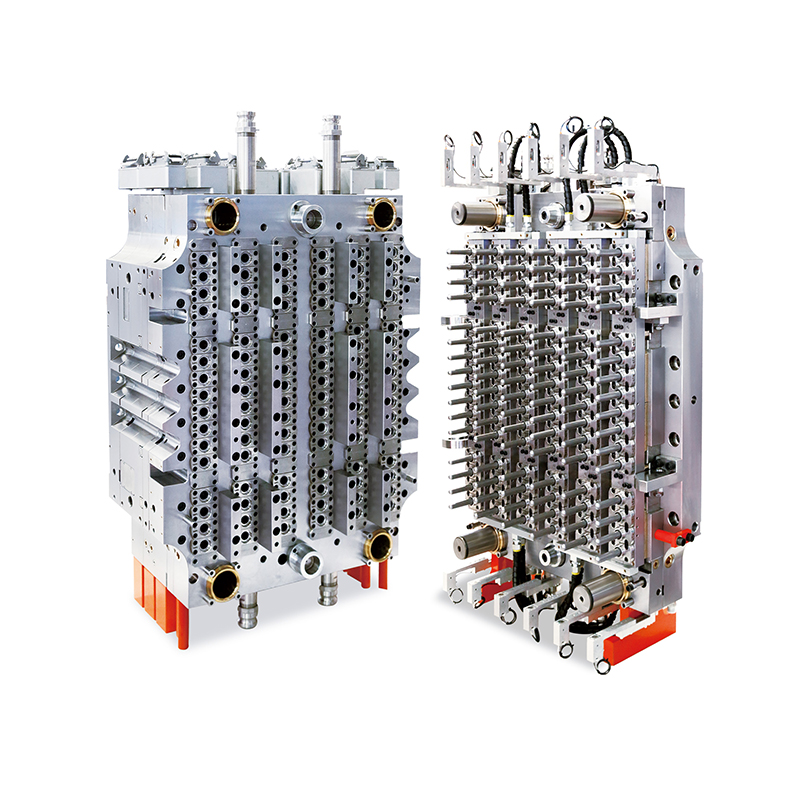
As global healthcare systems and diagnostic laboratories continue to evolve, the demand for high-quality, standardized consumables such as blood collection tubes is surging. One crucial component fueling this upward trend is the 48-Cavity Blood Collection Tube Mold, which has rapidly become an essential asset for manufacturers looking to scale their operations without compromising quality.
Designed to address the specific requirements of the medical device and diagnostics sectors, this high-output mold is redefining the way blood collection tubes are manufactured. Offering throughput, dimensional precision, and operational stability, the mold meets the growing needs of both centralized laboratories and point-of-care facilities worldwide.
Meeting the Demand for Medical-Grade Precision
Blood collection tubes are not ordinary plastic components—they must meet rigorous quality control standards. Any variance in wall thickness, length, or sealing surface can affect sample integrity or compromise testing accuracy. The 48-cavity mold addresses these issues head-on, delivering exceptionally consistent tube geometry across all cavities in every production cycle.
Each cavity is manufactured using CNC machining techniques with micron-level tolerances, ensuring that the final output meets international standards for vacuum integrity, labeling compatibility, and downstream automation. This level of precision is particularly vital for diagnostic labs using automated blood analysis equipment, where even a slight deviation can result in failed tests or equipment malfunction.
Engineered for Efficiency and Scalability
With the capability to produce 48 tubes in one injection molding cycle, the mold significantly increases production efficiency compared to lower-cavity designs. This boost in output is essential as healthcare providers face rising patient volumes and laboratories expand their test menus.
By reducing cycle times and material waste, the mold supports a lean manufacturing model while maintaining stringent quality control. This positions the 48-cavity blood tube mold as a critical tool for contract manufacturers and OEMs that need to meet high-volume purchase orders on tight timelines.
Advanced Mold Technologies Behind the Scenes
At the heart of this mold’s performance is a suite of engineering innovations. Many models feature hot runner systems with valve-gated nozzles, which allow for uniform material flow and reduced gate vestige—key in achieving bubble-free, optically clear tubes. These systems also eliminate the need for sprue trimming, resulting in cleaner, safer medical components straight out of the mold.
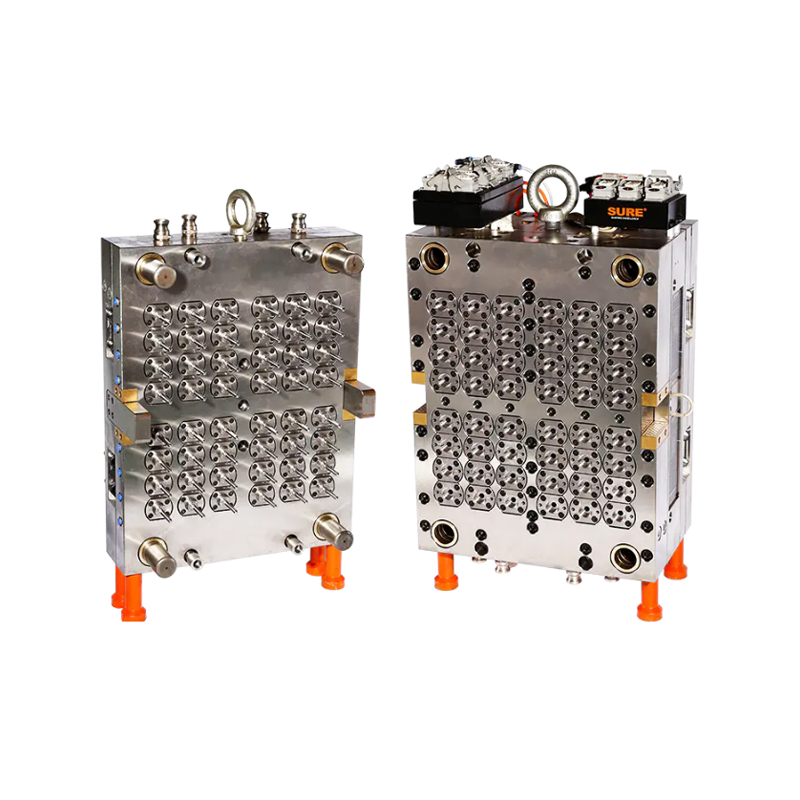
In addition, optimized cooling channels ensure that each preform cools evenly, minimizing shrinkage or warping. Temperature consistency across the mold prevents internal stresses that could later cause cracking or tube failure under vacuum conditions.
These design elements, when combined, result in better structural integrity, faster cycle times, and minimal part rejection—all while meeting ISO 13485 and FDA regulatory expectations.
Material Versatility and Application Flexibility
The 48-cavity blood collection tube mold can be customized to work with a range of medical-grade polymers, including polypropylene (PP), polyethylene terephthalate (PET), and other FDA-approved thermoplastics. This allows manufacturers to offer both traditional vacuum tubes and more specialized versions, such as plasma or serum separation tubes with additive coatings.
Moreover, the mold can be configured for various tube sizes and formats, including 13mm x 75mm or 16mm x 100mm variants, allowing producers to cater to diverse clinical and regional requirements without changing equipment.
Durability That Supports Continuous Production
Built using high-strength tool steels such as H13, S136, or 1.2083, the 48-cavity mold is engineered for extended operational life. These materials resist corrosion and abrasion from repeated molding cycles and contact with sterilizable medical resins.
For manufacturers running 24/7 operations, this translates to lower maintenance costs and fewer production interruptions. When paired with automated mold cleaning and maintenance protocols, the mold can maintain top-tier performance for millions of cycles.
Seamless Integration into Automated Environments
Modern blood tube manufacturing plants increasingly rely on automation. The 48-cavity mold is robotic handling compatible and integrates seamlessly with in-mold labeling systems, automated take-out units, and downstream packaging equipment. This enhances operational throughput while maintaining a sterile environment and reducing manual handling risks.
 英语
英语 法语
法语