No.148 Yongda Road, Jiangkou Street, Huangyan, Taizhou, Zhejiang, China.
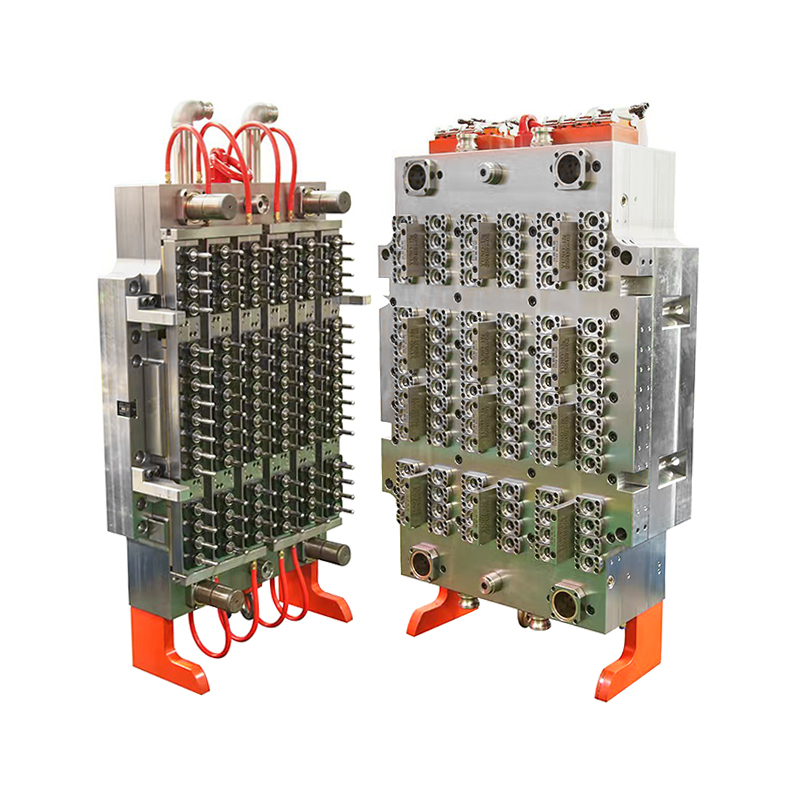
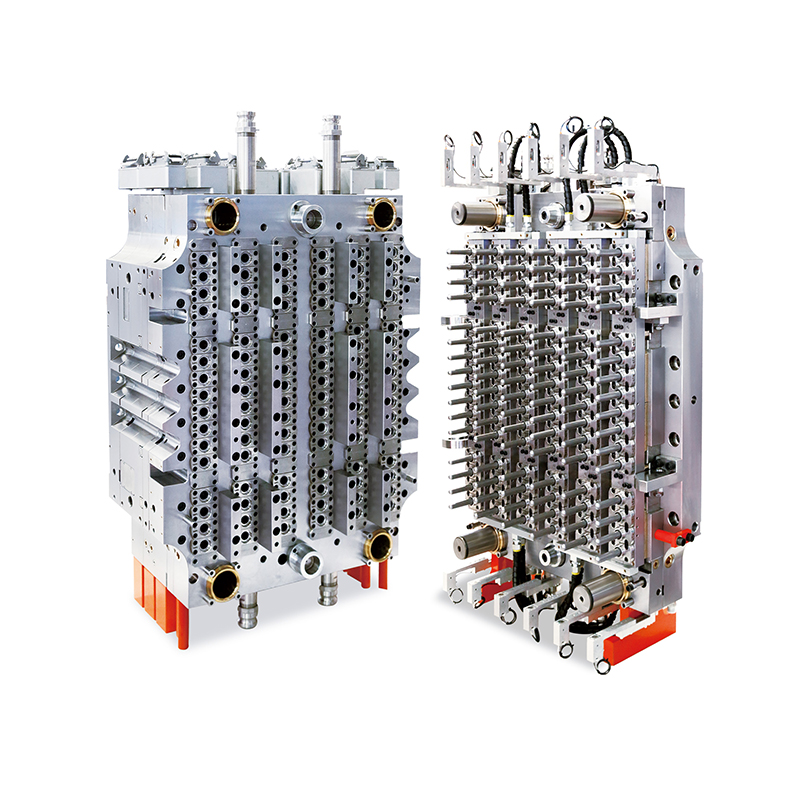
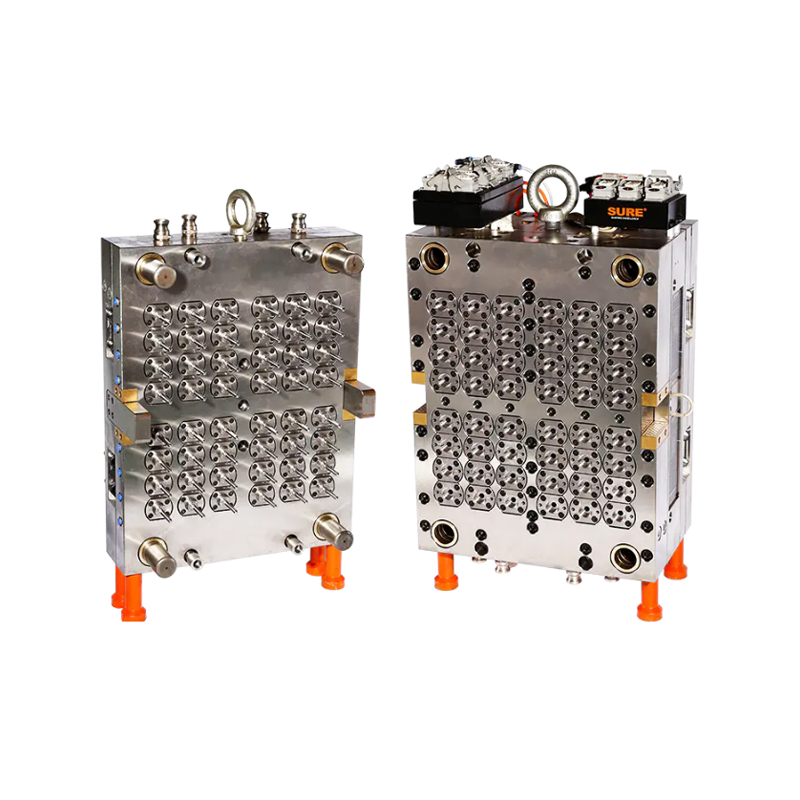
As the global biotechnology and life sciences industries continue their rapid evolution, the need for precision-engineered lab consumables has become more critical than ever. Among these essential tools is the virus transport tube—a core component used in specimen collection, virus storage, and molecular diagnostics. Responding to this growing demand, manufacturers are turning to the 32-Cavity Virus Tube Mold, a high-output production solution that is setting new benchmarks for efficiency, consistency, and scalability.
This mold is not only transforming how laboratories and diagnostic kit suppliers meet surging requirements, but it is also reinforcing the foundational role of quality tooling in public health and biomedical innovation.
Elevating Mass Production in Virus Transport Tube Manufacturing
The 32-cavity configuration offers a substantial increase in production volume compared to conventional lower-cavity molds. Capable of producing 32 virus tubes per injection cycle, this mold drastically improves manufacturing throughput without sacrificing dimensional accuracy or product integrity.
This capability is particularly significant for diagnostic and biotech companies that must rapidly scale up production during public health emergencies such as pandemic outbreaks or for ongoing global surveillance programs. By minimizing cycle time and material waste, the mold ensures efficient use of resources while meeting tight delivery deadlines.
Precision Engineering for Critical Applications
Unlike standard lab containers, virus transport tubes must adhere to stringent regulatory and dimensional specifications to ensure compatibility with testing platforms and cold chain logistics. The 32-Cavity Virus Tube Mold is engineered using high-tolerance machining techniques, delivering precise and repeatable results across all cavities.
Uniform wall thickness, smooth internal surfaces, and properly aligned caps and closures are essential to prevent contamination, leakage, or sample degradation. This level of quality is vital for preserving the accuracy of RT-PCR, antigen, and culture-based diagnostic methods, especially when samples are shipped over long distances or stored for extended periods.
Supporting Rapid Response and Pandemic Preparedness
During global health crises such as COVID-19 or outbreaks of emerging infectious diseases, supply chain bottlenecks in diagnostic components can severely hinder response efforts. The 32-cavity mold plays a key role in fortifying pandemic preparedness infrastructure, enabling faster production of high volumes of sample collection tubes used in testing kits.
Many governments and private laboratories are now investing in domestic capacity to produce virus transport mediums and containers. For these facilities, a mold that offers both high productivity and dependable quality is an invaluable asset. It supports local manufacturing efforts, reduces reliance on overseas suppliers, and helps meet sudden surges in demand without compromising quality or timelines.

Versatility Across Biotech Applications
While its primary use lies in virus transport tubes, the mold’s design also allows it to be adapted for various other biotech applications. These include cryovials, specimen storage tubes, reagent containers, and PCR sample vessels.
The mold accommodates various resins commonly used in biotech and medical-grade applications, such as polypropylene (PP) and polyethylene terephthalate (PET), which offer resistance to chemicals, low-temperature storage, and sterilization processes. Whether the tubes are destined for use in molecular diagnostics, vaccine production, or viral vector research, the 32-cavity mold provides the reliability and consistency necessary for sensitive biomedical workflows.
Durable Build for Long-Term, High-Volume Use
Crafted from premium-grade tool steels such as S136H or H13, the mold is designed to endure high-cavity stress and continuous production cycles. The materials are selected for their resistance to wear, corrosion, and thermal fatigue—factors that are especially important when working with medical-grade plastics and sterilizable environments.
The mold’s cooling channels are engineered to reduce cycle time and maintain dimensional stability, helping to preserve the tight tolerances required for automated filling and capping lines. The overall result is lower production downtime, fewer defects, and greater operational efficiency.
 英语
英语 法语
法语