No.148 Yongda Road, Jiangkou Street, Huangyan, Taizhou, Zhejiang, China.
In an age where scientific research and diagnostic testing are playing an increasingly pivotal role in global health and biotechnology, the tools that support these efforts must evolve to meet rising demand and precision standards. A innovation making waves in laboratory equipment manufacturing is the 4+4 Cavity Petri Dish Mold—a solution engineered to deliver consistency, speed, and product quality.
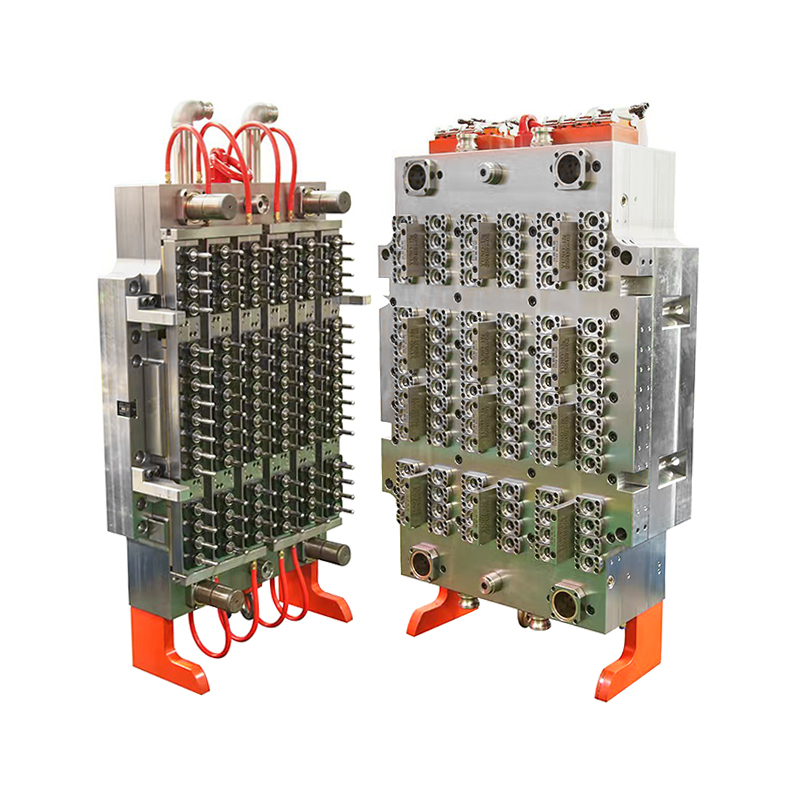
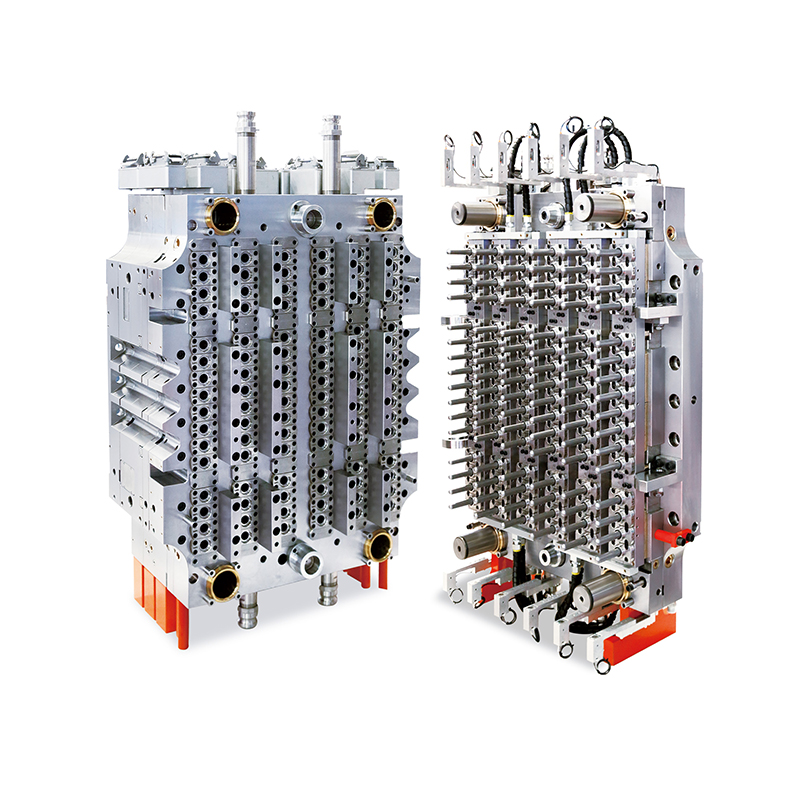
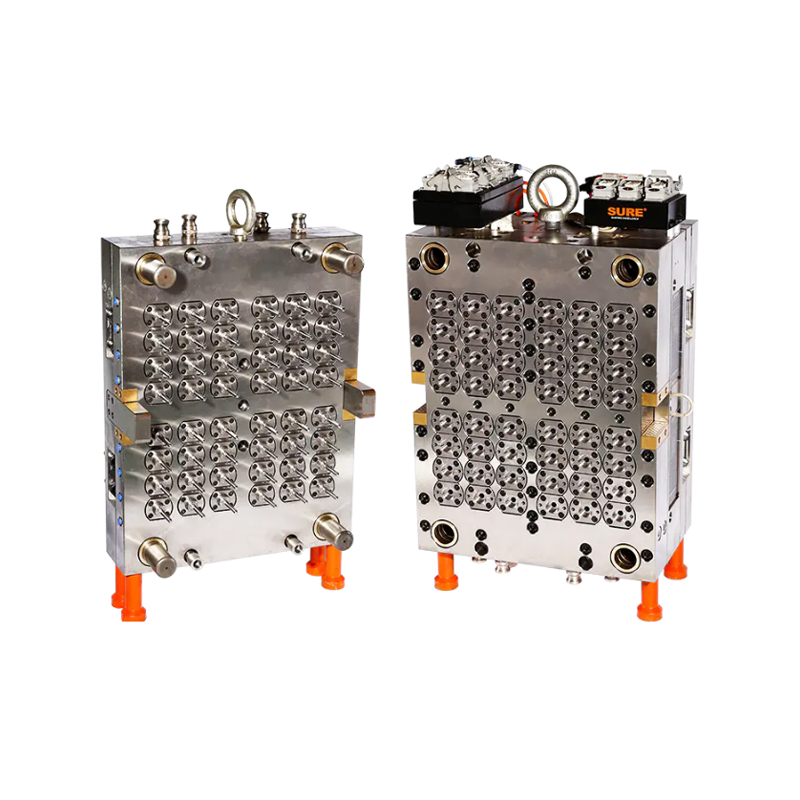
This mold has become a key asset for manufacturers producing sterile labware for hospitals, research institutions, and pharmaceutical companies. With its balanced dual-set configuration (4 base cavities + 4 lid cavities), the mold enables synchronized production of complete Petri dishes in a single cycle, streamlining operations and minimizing post-processing.
Addressing the Growing Demand for Laboratory Consumables
With increased global investment in microbiology, biotechnology, and pharmaceutical development, the demand for high-quality Petri dishes is surging. From cultivating microorganisms to analyzing tissue cultures, Petri dishes are foundational tools in countless experiments.
However, the challenge for manufacturers lies in delivering large volumes of labware without compromising precision or cleanliness. This is where the 4+4 cavity mold proves essential. By allowing for the simultaneous production of both dish bases and lids, it doubles productivity without increasing machine footprint, allowing suppliers to keep pace with laboratory needs.
Built for Reliability: Engineering That Supports Critical Research
In lab environments where even minute inconsistencies in equipment can skew results, the integrity of each Petri dish is non-negotiable. The 4+4 Cavity Mold is designed to eliminate such variability. It produces dishes with uniform thickness, optical clarity, and edge precision, ensuring reliable sealing, stacking, and usage under sterile conditions.
The mold's precision-engineered cavities help maintain exact shape tolerances, which is critical for applications involving agar mediums and microbial culture. This dimensional stability ensures a flat, level surface, reducing the risk of contamination or uneven bacterial growth.
High-Quality Materials for Sterile Environments
Another major advantage of this mold lies in its construction using high-performance tool steel and corrosion-resistant alloys. These materials are chosen not just for durability, but also for their compatibility with cleanroom and medical-grade environments. The mold can withstand repetitive high-temperature cycles and aggressive cleaning protocols commonly required in the production of sterile goods.

Its surface finishes are finely polished to reduce particle adhesion and improve mold release, helping ensure smooth product ejection and minimal manual handling, which are critical to maintaining sterility in final products.
Efficiency Through Design: Dual Production and Reduced Cycle Times
One of the features of the 4+4 cavity configuration is its inherent efficiency. Traditional single-part molds require separate cycles for lids and bases, effectively doubling the production time for a complete unit. This mold’s dual-plate setup eliminates that bottleneck.
By molding both halves of the Petri dish in one operation, the system significantly reduces total cycle time and energy consumption per unit. This increased throughput is especially beneficial for contract manufacturers and high-volume labware producers under tight delivery timelines.
In addition, the mold is designed with optimized cooling channels and balanced injection points, ensuring uniform cooling and material flow across all cavities. The result is shorter cycle durations and reduced product warping, which contributes to fewer rejects and lower operational costs.
Streamlining Quality Control and Automation Integration
With increasing adoption of automation in labware manufacturing, the mold’s consistency plays a critical role in downstream automation compatibility. Whether it’s robotic part handling, automated stacking, or sterile packaging systems, the uniformity of parts produced by the 4+4 mold reduces the chances of mechanical errors and misalignment.
It’s also compatible with vision inspection systems used to detect microscopic defects, thanks to the clarity and dimensional accuracy of the molded products. This simplifies quality assurance processes and minimizes batch loss, which is particularly important when producing labware destined for regulated medical markets.
 英语
英语 法语
法语