No.148 Yongda Road, Jiangkou Street, Huangyan, Taizhou, Zhejiang, China.
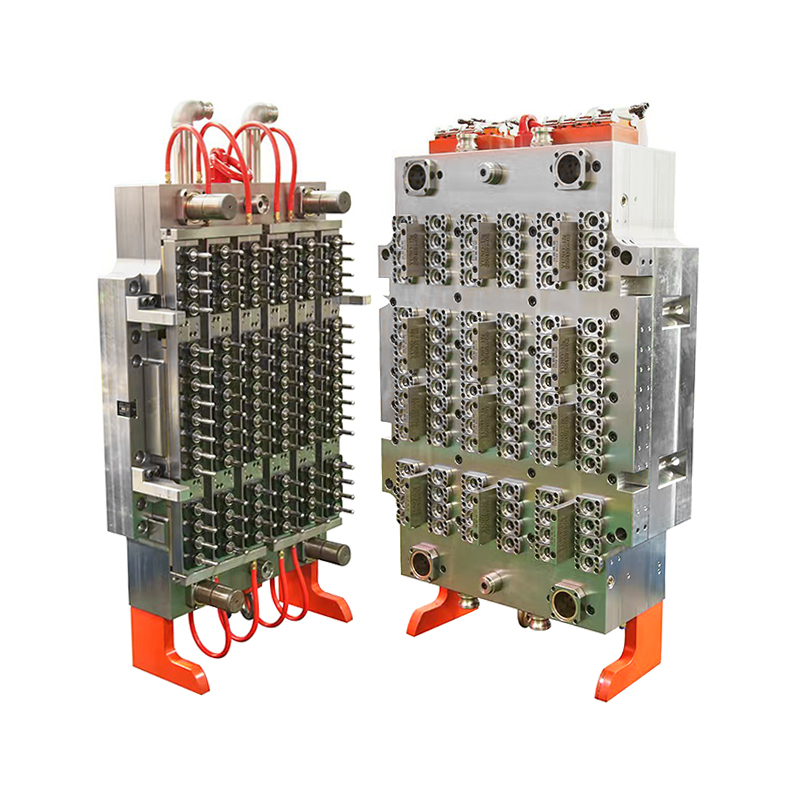
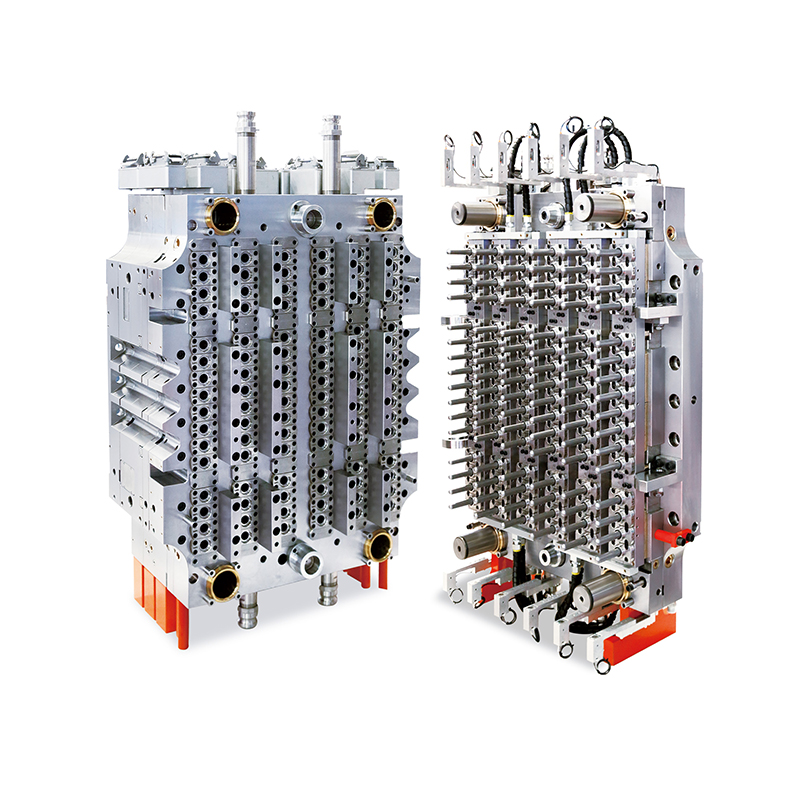
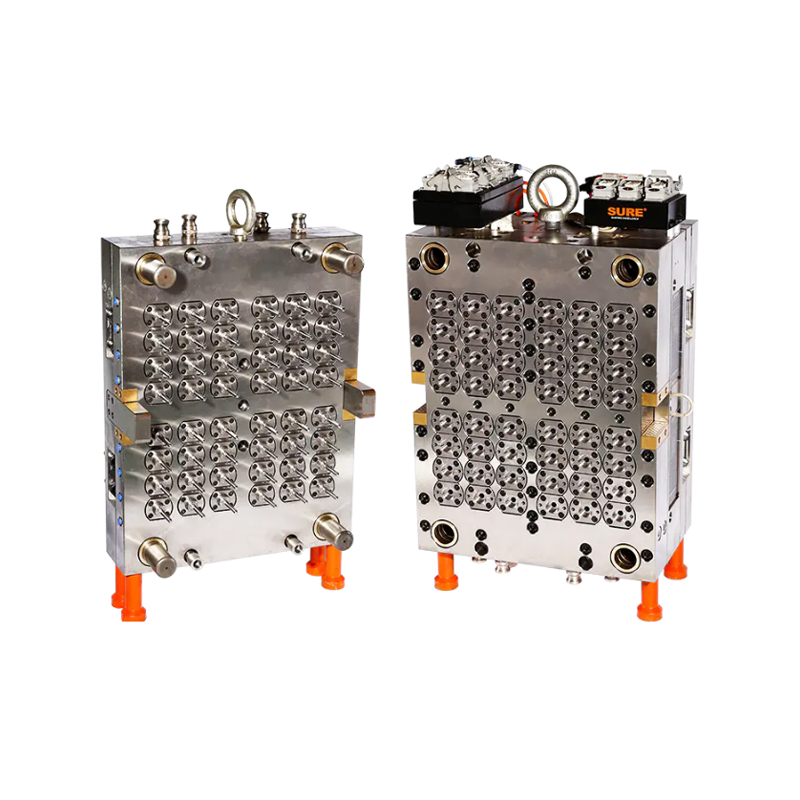
In an era where precision and efficiency are crucial for healthcare diagnostics and laboratory testing, the production of high-quality medical consumables has never been more vital. Among the essential items used in blood diagnostics is the blood collection tube—a product that must meet standards of safety, sterility, and dimensional accuracy. In response to these demands, manufacturers are increasingly adopting the 32-Cavity Blood Collection Tube Mold, a robust and efficient solution designed for high-volume, high-precision production.
This advanced mold is making a significant impact on medical device manufacturing by offering a practical balance of production capacity, reliability, and design precision, particularly in the context of strict global healthcare regulations and the growing demand for diagnostic supplies.
Increasing Demand for Diagnostic Consumables
Over the past several years, global healthcare systems have seen a sharp increase in the use of diagnostic tools. Routine blood tests, disease monitoring, and emergency health responses all rely heavily on reliable specimen collection tools. As a result, blood collection tubes are in high demand across hospitals, clinics, laboratories, and research centers.
To meet this increasing need without compromising on quality or compliance, manufacturers are turning to high-performance molds like the 32-cavity design, which can efficiently produce 32 tubes in each injection cycle—a major boost to production speed and output consistency.
Advanced Tooling for Precision and Consistency
The medical industry requires consumables that are both sterile and dimensionally precise. Blood collection tubes must be uniformly shaped to ensure compatibility with needles, caps, centrifuges, and analyzers. Inconsistencies can lead to leaks, measurement errors, or mechanical issues during analysis.
The 32-Cavity Blood Collection Tube Mold is engineered to achieve exceptionally tight tolerances in every cycle. Its advanced cavity design and precise cooling channels ensure that each tube features smooth surfaces, consistent wall thickness, and accurate dimensions—factors critical for proper sample containment and lab equipment compatibility.
Moreover, the mold is designed for use with medical-grade plastics, such as PET and polypropylene, ensuring biocompatibility and clarity while allowing for downstream processes like labeling, sterilization, and packaging.
Built for Long-Term, Reliable Performance
One of the features of the 32-cavity mold is its robust construction. Unlike conventional molds that may degrade over time due to chemical exposure or repeated use, this mold is built using industrial-grade, high-performance materials.

Its key components are crafted from stainless steel with enhanced hardness and surface treatments, offering resistance to wear, thermal stress, and chemical corrosion. These properties are particularly important in environments where molds are subjected to high temperatures and aggressive cleaning agents.
This level of durability means less frequent maintenance, fewer shutdowns, and a longer operational lifespan, making it a cost-effective investment for manufacturers with high output requirements.
Cleanroom Compatibility and Sterile Manufacturing
Manufacturing blood collection tubes requires adherence to stringent cleanliness standards. The 32-Cavity Blood Collection Tube Mold is optimized for use in cleanroom environments compliant with ISO Class 7 or 8 standards, allowing for the production of sterile or sterile-ready components.
Additionally, the mold can be paired with automated systems for part removal, quality inspection, and packing, further reducing human contact and the risk of contamination. This capability aligns with global practices in the production of Class I and Class II medical devices, helping manufacturers maintain certifications such as ISO 13485 and FDA registration.
Streamlining Production and Reducing Waste
The mold design incorporates efficient hot or cold runner systems, which allow for reduced material waste and consistent resin flow to each cavity. This results in uniform filling, minimal scrap rates, and optimized cycle times. The improved material utilization supports cost-saving efforts while also contributing to more sustainable manufacturing operations.
Furthermore, mold makers often offer modular configurations, allowing manufacturers to easily switch cavity types, adjust tube lengths, or integrate new features, such as color-coded caps or safety closures, based on market needs.
 英语
英语 法语
法语